推荐产品
公司新闻/正文
热烈祝贺上海信帆客户又一篇SCI文章被国际知名期刊收录
人阅读 发布时间:2017-04-19 11:55
热烈祝贺上海信帆客户又一篇SCI文章被国际知名期刊收录
热烈祝贺我司客户又一篇SCI文章被国际知名期刊收录
祝贺我司客户的优质研究成果被国际知名期刊《Clinical Rheumatology》收录

该论文标题: Predictors of response to TNFα antagonist therapy in Chinese rheumatoid arthritis
Clinical Rheumatology
发表日期:July 2015, Volume 34
期刊号:Issue 7, pp 1203–1210
老师在论文后期编辑过程中与我们积极互动,信帆生物给予了全方位支持,最终顺利被《Clinical Rheumatology》录用。
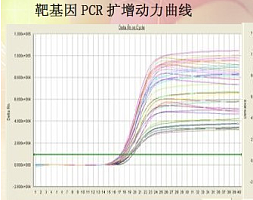
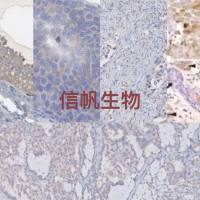
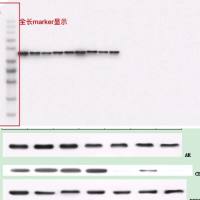
以下是老师论文中使用我司产品的截图
该论文中所使用的以下ELISA试剂盒均购自我司。
Human IL-34 ELISA KIT(Xinfan Biotechology Co.,Ltd,shanghai,China)
Human TNF-a ELISA KIT(Xinfan Biotechology Co.,Ltd,shanghai,China)
Human IL-6 ELISA KIT(Xinfan Biotechology Co.,Ltd,shanghai,China)
Human IL-8 ELISA KIT(Xinfan Biotechology Co.,Ltd,shanghai,China)
Human MMP-3 ELISA KIT(Xinfan Biotechology Co.,Ltd,shanghai,China)
信帆生物是一家专业的elisa试剂盒供应商,销售各种进口国产品牌的elisa试剂盒。
对于elisa试剂盒,我们提供完善的质量保证,专业的技术解答,耐心周到的售后服务,成为国内多家科研机构和高校的试剂盒指定供应商。
信帆生物从创立至今,具有多年试剂领域的研发、生产和销售经验,目前已经成长为国内规模较大的试剂研发、生产和销售的综合性企业,一直为科研工作者助力。我们期待您的光临!
热烈祝贺上海信帆客户又一篇SCI文章被国际知名期刊收录
《Clinical Rheumatology》期刊介绍:
Clinical Rheumatology is an international journal devoted to publishing in the English language original clinical investigation and research in the general field of rheumatology with accent on clinical aspects at postgraduate level. Studies carried out anywhere in the world will be considered the basic criterion for acceptance being the medical and scientific standard of the work described.
文章介绍
This study aimed to investigate the clinical, immunological, and radiologic predictors of response to tumor necrosis factor (TNF)-α antagonist therapy in Chinese rheumatoid arthritis (RA). Ninety RA patients were divided into two groups according to their responsiveness to TNF-α antagonist therapy at 1 month: group A (responders) and group B (non-responders). After 3 months of therapy, all the 90 patients were re-assessed and re-divided into another two groups: group C (responders) and group D (non-responders). Serum samples and clinical characteristics as well as radiographic features were collected at baseline, first month, and third month post-initial administration of TNF-α antagonist. Serum TNF-α, interleukin (IL)-6, IL-8, IL-34, and matrix metalloproteinase (MMP)-3 were measured by enzyme-linked immunosorbent assay (ELISA). Disease activity and Sharp score were evaluated. (1) Comparisons between groups A and B: subjects in group A showed a lower level of erythrocyte sedimentation rate (ESR) and a higher level of albumin (ALB) at baseline than that of group B (p < 0.05). The cutoff value of ALB for prediction was ≥34.9 g/l and that of ESR was ≤55.5 mm/h. (2) Comparisons between groups C and D: group C showed lower levels of ESR, health assessment questionnaire (HAQ), and IL-34 at baseline (p < 0.05). The threshold for prediction were as follows: ESR ≤60 mm/h, HAQ ≤1.3125, and IL-34 ≤194.12 pg/ml. (3) The serum cytokines were positively correlated with C-reactive protein (CRP) and disease activity index, while ALB was negatively correlated with CRP and disease activity. Baseline ALB ≥34.9 g/l or ESR ≤55.5 mm/h might predict a good response at 1-month treatment of TNF-α antagonist, while baseline ESR ≤60 mm/h, HAQ ≤1.3125, and IL-34 ≤194.12 pg/ml might predict a good response at 3-month treatment.
热烈祝贺上海信帆客户又一篇SCI文章被国际知名期刊收录